Abstract
Introduction: CCAAT-enhancer-binding protein α (C/EBPα) is one of the most essential lineage-specific transcription factors involved in myeloid differentiation and includes two isoforms of protein: full-length C/EBPα-p42 and truncated C/EBPα-p30. Acute myeloid leukemia (AML) with CEBPA biallelic (CEBPAbi) mutation or single mutation in the bZIP region is associated with a favorable prognosis, but the mechanisms are still unclear. The unfolded protein response (UPR) is a conserved adaptive signal transduction pathway triggered by endoplasmic reticulum (ER) stress. UPR first strives to restore protein homeostasis and maintain cell survival through a pro-survival arm called adaptive UPR. However, in the setting of irremediable ER stress, the UPR transforms into pro-apoptotic terminal UPR signaling that promotes cell apoptosis. DNA damage-inducible transcript 3 (DDIT3) is a key transcription factor responsible for terminal UPR under severe ER stress. Here, we hypothesize that C/EBPα two isoforms regulate DDIT3 transcription, resulting in easier elimination of AML cells with CEBPA mutations and dysregulation by terminal UPR.
Methods: Human AML cell lines and mouse 32Dcl3 were transduced with recombinant lentiviruses to knockdown CEBPA expression or over-express of C/EBPα two isoforms. RT-qPCR was used to detected the mRNA levels and western immunoblotting was used to determine the protein levels. The cell apoptosis rates were determined as the percentage of Annexin V-positive cells by flow cytometry. We performed CFU assays to confirm the declined granulocytic differentiation of 32Dcl3 cells with Cebpa knockdown. To identify potential C/EBPα-binding DNA sequences in the genome, we performed ChIP-seq. Luciferase reporter analysis was used to confirm the transcriptional regulation ability of C/EBPα two isoforms and independent ChIP-qPCR of candidate sequences were performed to further verify the transcription factor binding sites of C/EBPα. Finally, we generated a xenograft model with NCG mice to determine the potential application value of our findings.
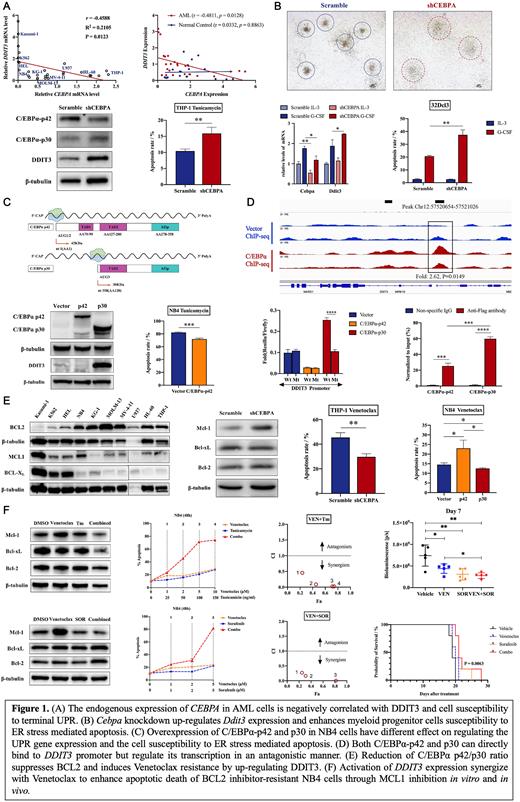
Results: Firstly, we found a negative correlation between CEBPA expression and DDIT3 levels in AML cell lines and primary de novo AML patients. After knockdown of CEBPA in THP-1 cells, which has high endogenous C/EBPα expression, DDIT3 expression was upregulated, and an increased apoptotic rate of AML cells was induced by ER stress. Cebpa knockdown also induced easier elimination of mouse myeloid progenitor cells 32Dcl3 due to upregulation of Ddit3, so as to avoid leukemogenesis since their differentiation was blocked. After the overexpression of C/EBPα-p42 and C/EBPα-p30 in NB4 cells, which have relatively low levels of endogenous C/EBPα isoforms, we found that C/EBPα-p30 activated DDIT3 expression to increase the sensitivity of AML cells to tunicamycin. However, C/EBPα-p42 had the opposite effect to C/EBPα-p30. Through luciferase reporter analysis, we confirmed that C/EBPα-p30 and C/EBPα-p42 directly regulate the transcription of DDIT3 in a contrasting manner, indicating that the two isoforms maintain protein homeostasis corporately. ChIP-seq and ChIP-qPCR revealed that C/EBPα-p30 has a special binding site in DDIT3 gene promoter with stronger affinity than C/EBPα-p42 (hg38, Chr12:57520654-57521026). Given that the BCL2 family proteins are major targets of DDIT3, we further observed that a low p42/p30 ratio was related to reduced BCL2 levels-a major target of DDIT3, increased MCL1 levels, and resistance of AML cells to venetocalx. Finally, in vitro and in vivo we found that tunicamycin and sorafenib could synergistically reverse venetoclax resistance in AML cells by activating the terminal UPR and inhibiting MCL1.
Conclusions: Collectively, our results identified a critical regulation of C/EBPα two isoforms on the transcription of DDIT3 in AML cells as well as normal myeloid progenitor cells and confirmed the importance of this regulation in leukemia surveillance and elimination of AML cells. Moreover, we demonstrated that patients with AML having a low C/EBPα p42/p30 ratio and CEBPAbi mutation may not benefit from monotherapy with BCL2 inhibitors. This can be resolved by a combination of ER stress inducers or MCL1 inhibitors, including tunicamycin and sorafenib, which may represent a novel therapeutic strategy for AML patients with CEBPA mutations and dysregulation.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal